- Memorandum of Agreement (MOA)
- Clinical Trial Agreement (CTA)
- Research Fund Agreement (RFA)
Drafting a Research Collaboration Agreement with the Funding Agency/Sponsor
Research Collaboration Agreement
Research Collaboration Agreement (RCA) is a general term for legally binding agreements between the University of the Philippines and one or more institutions in the conduct of a research project or program. CRAs define the relationship between the parties and should contain the following information: 1) background and objectives of the research, 2) name of the principal investigator and the research generating unit, 3) financial support, 4) scope of work, 5) intellectual property, 6) dispute resolution, 7) indemnification, 8) confidentiality and 9) use of the name of the University in all matters relating to the research. Examples of CRAs are Memorandum of Agreements (MOA), Memorandum of Understanding (MOU) and Clinical Trial/Study Agreements (CTA/CSA).
General Guidelines for Research Collaboration Agreements
All sponsored researches to be performed by any faculty, REPS or student of the University of the Philippines Manila , should be registered and undergo technical and ethical review prior to implementation. The implementation of these trials will be governed by the approved protocol and an executed collaborative research agreement (CRA), as reviewed by the UP Manila Legal Office and approved by the Chancellor or UP President.
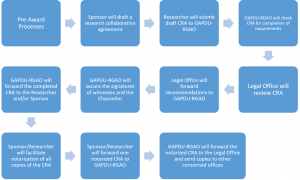
Figure 1: Steps for Research Collaborative Agreements
Processing of Collaborative Research Agreements
- Once registered, Researchers who have registered clinical trials will receive the UP Manila Legal Office Checklist of Liability and Indemnity Provision in Clinical Trial Agreements (Annex) and the Checklist of Documentary Support for the Endorsement of Clinical Trial Agreements (Appendix) in their e-mail. Researchers of sponsored non-clinical trials will receive the UP Manila Legal Office Checklist for Memorandum of Agreement (Annex) or the Memorandum of Agreement template (Annex).
2.The sponsor or clinical research organization (CRO) will draft an appropriate CRA according to the checklist and sign it. They will submit five (5) originally signed drafts along with the required documentary support to the GAPDU for processing.
3.The GAPDU personnel in charge will check the documents for completion. If it is complete, s/he will forward the draft and the attachments to the UP Manila Legal Office. at the 8th floor of the UP-PGH building.
4. The Chief Legal Officer will review the draft CRA and request for additional changes and documents, if necessary. He will then return the draft, with comments, to the GAPDU.
5. If there are additional changes or documents that need to be provided, the GAPDU staff will return the draft CRA with the Chief Legal Officer’s comments for compliance.
6. Once the appropriate changes have been made or the additional documents provided, the GAPDU staff will submit the draft CRA once more to the UP Manila Legal Office.
7. The Chief Legal Officer will review the revised CRA and documents once more. Once s/he deems that all the terms and conditions are in order, s/he will countersign the lower right hand corner of all pages of the CRA and will likewise sign besides the space provided for the Chancellor’s signature. The signed documents will then be forwarded back to the GAPDU to secure all other required signatures of University officials.
8. If one of the witnesses is the PGH Hospital Director, the GAPDU Head will create a Reference Slip (Appendix) which contains the following additional information:
a. Total Budget
b. Honoraria of the Principal Investigator
c. Administrative Overhead Cost breakdown
9. Once all signatories have signed, the GAPDU staff will endorse the signed agreement to the sponsor/researcher to facilitate notarization of the CRAs.
10. The notarized CRA will be scanned and kept in a secure online cloud storage for safe-keeping. Copies will be provided to the following units:
a. UP Manila Legal Office
b. Philippine General Hospital (if applicable)
c. Research Grants Administration Office (FAMU)
d. UP Manila Accounting Office
e. UP Manila Internal Audit
